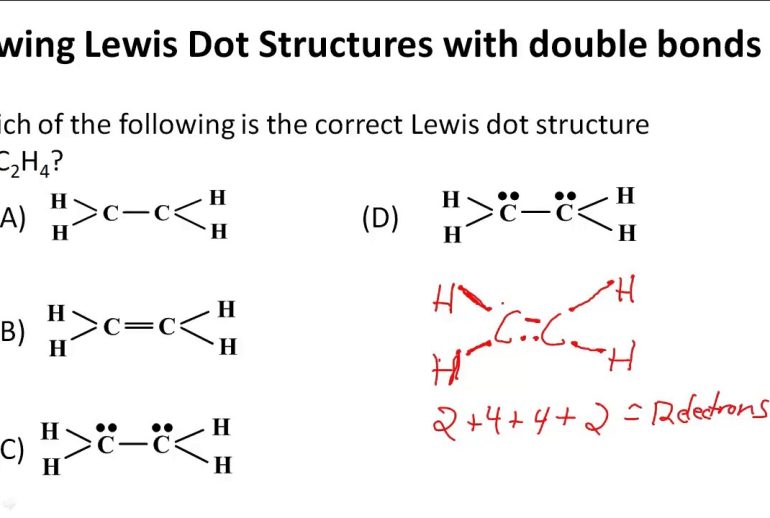
To know when to draw a double bond in a Lewis structure, look for certain signs. Check the number of available electrons and the need to satisfy the octet rule.
Understanding Lewis structures is crucial in chemistry. They help us visualize molecules and their bonds. But sometimes, deciding between single and double bonds can be tricky. Double bonds often appear when single bonds can’t satisfy the atoms’ electron needs. Recognizing these patterns helps in drawing accurate structures.
This blog will guide you through the signs and rules for drawing double bonds. Let’s make Lewis structures easier to understand.
Lewis Structures Basics
Understanding Lewis structures is key in chemistry. These diagrams show how atoms share electrons. They help us predict molecule shapes and reactions. Let’s dive into the basics of Lewis structures.
Octet Rule
The octet rule is simple. Most atoms want eight electrons in their outer shell. This makes them stable. Think of it as a rule of thumb. For example, oxygen has six valence electrons. It needs two more to reach eight. Drawing a double bond helps oxygen achieve this.
Electron Dot Diagrams
Electron dot diagrams show valence electrons. Each dot represents one electron. Start with the central atom. Then, add the other atoms around it. Count all the valence electrons. Place dots around atoms to show bonds. Sometimes, single bonds are not enough. You might need double bonds to satisfy the octet rule.

Bond Types
Understanding bond types is key in drawing Lewis structures. Bonds can either be single, double, or triple. This section will focus on single and double bonds.
Single Bonds
Single bonds are the most basic type. They involve two electrons. One electron comes from each atom. In a single bond, atoms share one pair of electrons. This bond type is often found in molecules like hydrogen (H2) and methane (CH4).
Single bonds are represented by a single line between two atoms. They are easy to spot in Lewis structures. This bond is the simplest and most common. It allows for free rotation around the bond axis.
Double Bonds
Double bonds involve four electrons. Each atom shares two electrons. This means they share two pairs of electrons. Double bonds are stronger than single bonds. They restrict rotation around the bond axis.
Double bonds are represented by two lines between the atoms. They are often found in molecules like oxygen (O2) and carbon dioxide (CO2). Double bonds are important in determining the shape and reactivity of a molecule.
Electron Pair Sharing
Understanding electron pair sharing is crucial in drawing Lewis structures. Electrons can either be shared between atoms or remain unshared. Shared electrons form bonds, which can be single, double, or triple bonds. Knowing when to draw a double bond is key to accurate representations.
Bonding Pairs
Bonding pairs are electrons shared between atoms. They form bonds and hold the atoms together. In a Lewis structure, a single line represents a single bond. A double bond uses two lines, showing two pairs of shared electrons. Double bonds often occur between carbon, oxygen, and nitrogen atoms.
Consider the molecule carbon dioxide (CO2). Carbon shares two pairs of electrons with each oxygen. This results in two double bonds. Each double bond is shown with two lines in the Lewis structure. Double bonds are shorter and stronger than single bonds.
Lone Pairs
Lone pairs are electron pairs not shared between atoms. They belong to a single atom and do not form bonds. In Lewis structures, lone pairs are shown as pairs of dots. These pairs influence the shape and reactivity of molecules.
Take the water molecule (H2O) as an example. Oxygen has two lone pairs of electrons. These lone pairs push the hydrogen atoms closer, creating a bent shape. Lone pairs can also affect the need for double bonds. If there are not enough lone pairs, a double bond might be necessary to satisfy the octet rule.

Formal Charge Calculation
Understanding when to draw a double bond in a Lewis structure can be tricky. One essential method involves formal charge calculation. This technique helps determine the most stable structure. It ensures that atoms have the lowest possible charges.
Charge Minimization
Charge minimization is key in formal charge calculation. Each atom should have a formal charge close to zero. This minimizes the overall charge of the molecule. To find the formal charge, use the formula:
Formal Charge = Valence Electrons – (Non-bonding Electrons + 1/2 Bonding Electrons)
If the formal charge is high, consider adding a double bond. This can reduce the charge on atoms. It leads to a more stable Lewis structure. Always aim for the smallest possible formal charges.
Structure Validity
Structure validity ensures the Lewis structure is correct. Valid structures follow the octet rule. This means atoms strive for eight electrons in their valence shell. Sometimes, adding a double bond helps meet this rule.
Check all atoms in the structure. Ensure they have complete octets. If not, adjust by adding double bonds where necessary. This creates a stable and valid Lewis structure. A valid structure reduces the overall energy of the molecule.
By using formal charge calculation, you can confidently draw double bonds. This leads to more accurate and stable Lewis structures.
Resonance Structures
Understanding resonance structures is key in drawing Lewis structures. Resonance structures are different ways to represent a molecule. They show possible electron arrangements within a molecule. These structures help predict the actual distribution of electrons. Resonance is essential when a single Lewis structure cannot describe a molecule.
Equivalent Forms
Resonance structures often have equivalent forms. These forms depict the same molecule. The only difference is the position of electrons. Each form contributes to the overall structure. This helps create a more accurate picture of the molecule.
For example, ozone (O3) has two resonance structures. Both forms show a double bond, but in different places. This indicates the double bond is delocalized. It means the electrons spread over more than one bond.
Stabilization
Resonance structures help stabilize molecules. They show how electrons can move within a molecule. Delocalized electrons can lower the energy of the molecule. This makes the molecule more stable.
Benzene (C6H6) is a good example. It has six carbon atoms forming a ring. Each carbon-carbon bond is a mix of single and double bonds. The resonance structures show the electrons shared equally. This equal sharing adds stability to benzene.
Exceptions To The Octet Rule
When drawing Lewis structures, the octet rule is a helpful guideline. It states that atoms strive to have eight electrons in their outer shell. Yet, there are exceptions to this rule. Some molecules do not follow the octet rule and require a different approach. Understanding these exceptions will help you draw accurate Lewis structures.
Expanded Octet
Some elements can have more than eight electrons in their valence shell. This is known as an expanded octet. Usually, this occurs with elements in the third period or beyond. Phosphorus, sulfur, and chlorine are common examples. They have empty d-orbitals that can hold extra electrons. So, if you see these elements, consider an expanded octet.
Incomplete Octet
Some elements can have fewer than eight electrons in their valence shell. This is called an incomplete octet. Hydrogen, helium, lithium, and beryllium are typical examples. They are stable with fewer than eight electrons. For instance, hydrogen is stable with two electrons. Beryllium often has four electrons in its stable state.
Common Mistakes
Creating accurate Lewis structures can be tricky. Many students make common mistakes. Understanding these errors helps improve your skills. Let’s explore two frequent pitfalls: overlooking electrons and incorrect bonding.
Overlooking Electrons
One common mistake is overlooking electrons. Every atom must have the correct number of electrons. Missing electrons can lead to incorrect structures. Always count total valence electrons. This count must match the sum of all atoms’ valence electrons. Failing to do this can create errors in your Lewis structure.
Also, remember lone pairs. Lone pairs are non-bonding electrons. They play a crucial role in a molecule’s shape. Forgetting lone pairs can result in wrong predictions. Ensure each atom has the right number of lone pairs. This helps in drawing the correct structure.
Incorrect Bonding
Incorrect bonding is another frequent mistake. Double bonds are important in many molecules. Knowing when to draw them is key. Some students mistakenly draw single bonds where double bonds are needed. This mistake changes the molecule’s properties.
To avoid this, consider the octet rule. Most atoms seek to have eight electrons in their outer shell. Double bonds help achieve this in some cases. Also, check formal charges. Atoms with zero formal charge are more stable. Double bonds can help in achieving this stability.

Frequently Asked Questions
What Is A Double Bond In A Lewis Structure?
A double bond shares two pairs of electrons between atoms, making the bond stronger.
How Can You Identify When To Draw A Double Bond?
Look for atoms that need more electrons to complete their valence shells.
Why Are Double Bonds Important In Lewis Structures?
Double bonds help achieve the correct number of electrons for each atom, ensuring stability.
When Should You Not Draw A Double Bond?
Avoid double bonds when atoms already have complete valence shells with single bonds.
Conclusion
Knowing when to draw a double bond in a Lewis structure is vital. It helps you show the correct molecule structure. Double bonds appear when atoms share two pairs of electrons. This often happens with carbon, oxygen, or nitrogen. Check the total electrons and use them wisely.
Practice drawing Lewis structures to become better. Remember, practice makes perfect. Keep learning and applying these tips. Your chemistry skills will grow stronger. Happy drawing!